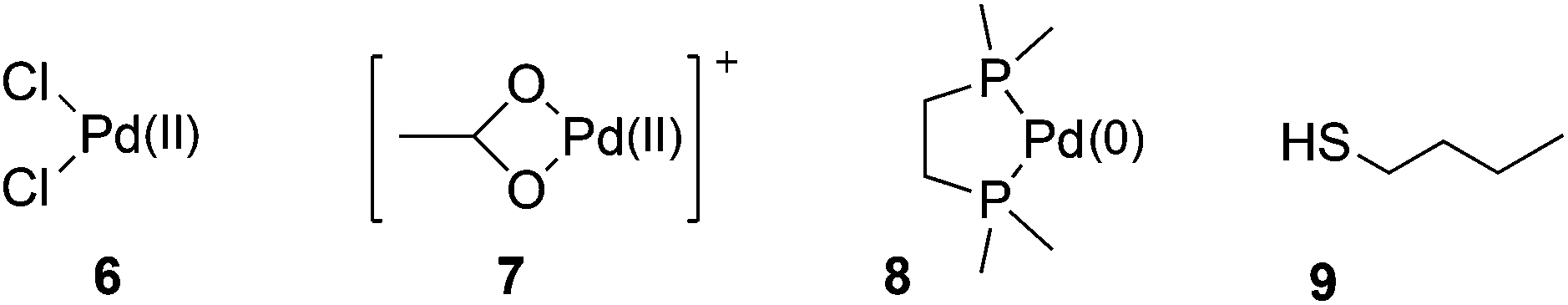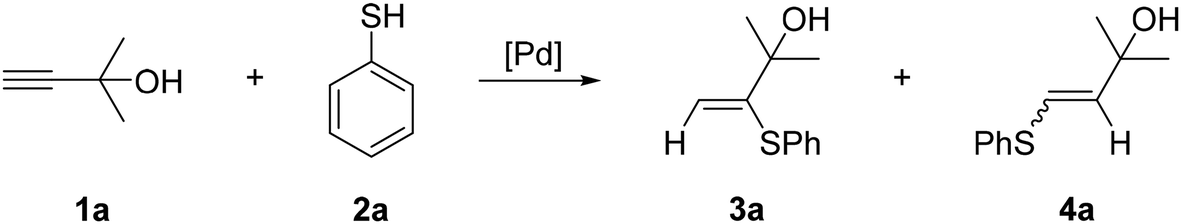
Ten-fold boost of catalytic performance in thiol–yne click reaction enabled by a palladium diketonate complex with a hexafluoroacetylacetonate ligand - Catalysis Science & Technology (RSC Publishing)
![Mixed ligand complexes of [Pd(terpy)(H2O)]2+ with some selected amino acids, peptides, DNA and related ligands - ScienceDirect Mixed ligand complexes of [Pd(terpy)(H2O)]2+ with some selected amino acids, peptides, DNA and related ligands - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S1878535214002652-gr2.jpg)
Mixed ligand complexes of [Pd(terpy)(H2O)]2+ with some selected amino acids, peptides, DNA and related ligands - ScienceDirect

Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes - ScienceDirect
Synthesis of Alkanethiolate-Capped Metal Nanoparticles Using Alkyl Thiosulfate Ligand Precursors: A Method to Generate Promising
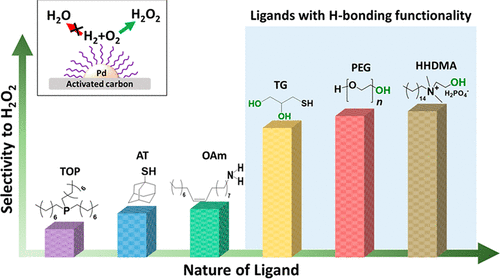
Tunable Catalytic Performance of Palladium Nanoparticles for H2O2 Direct Synthesis via Surface-Bound Ligands - ACS Catal. - X-MOL

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Pd/BIPHEPHOS is an Efficient Catalyst for the Pd‐Catalyzed S‐Allylation of Thiols with High n‐Selectivity - Schlatzer - 2020 - Advanced Synthesis & Catalysis - Wiley Online Library

Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes - ScienceDirect

Effects of two different thiol ligands to form a mixed monolayer ligand... | Download Scientific Diagram
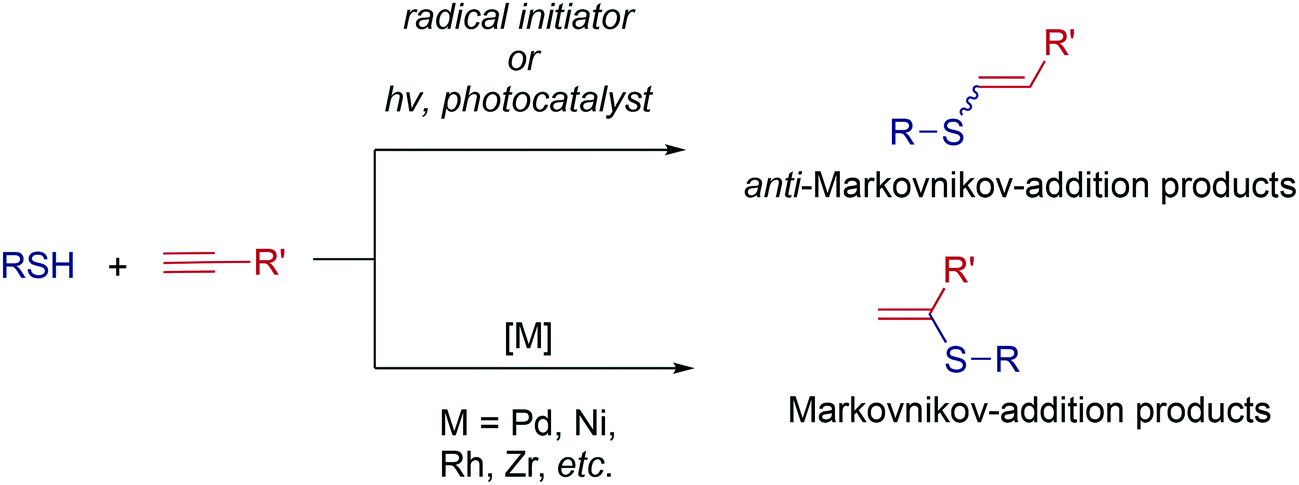
Ten-fold boost of catalytic performance in thiol–yne click reaction enabled by a palladium diketonate complex with a hexafluoroacetylacetonate ligand - Catalysis Science & Technology (RSC Publishing)
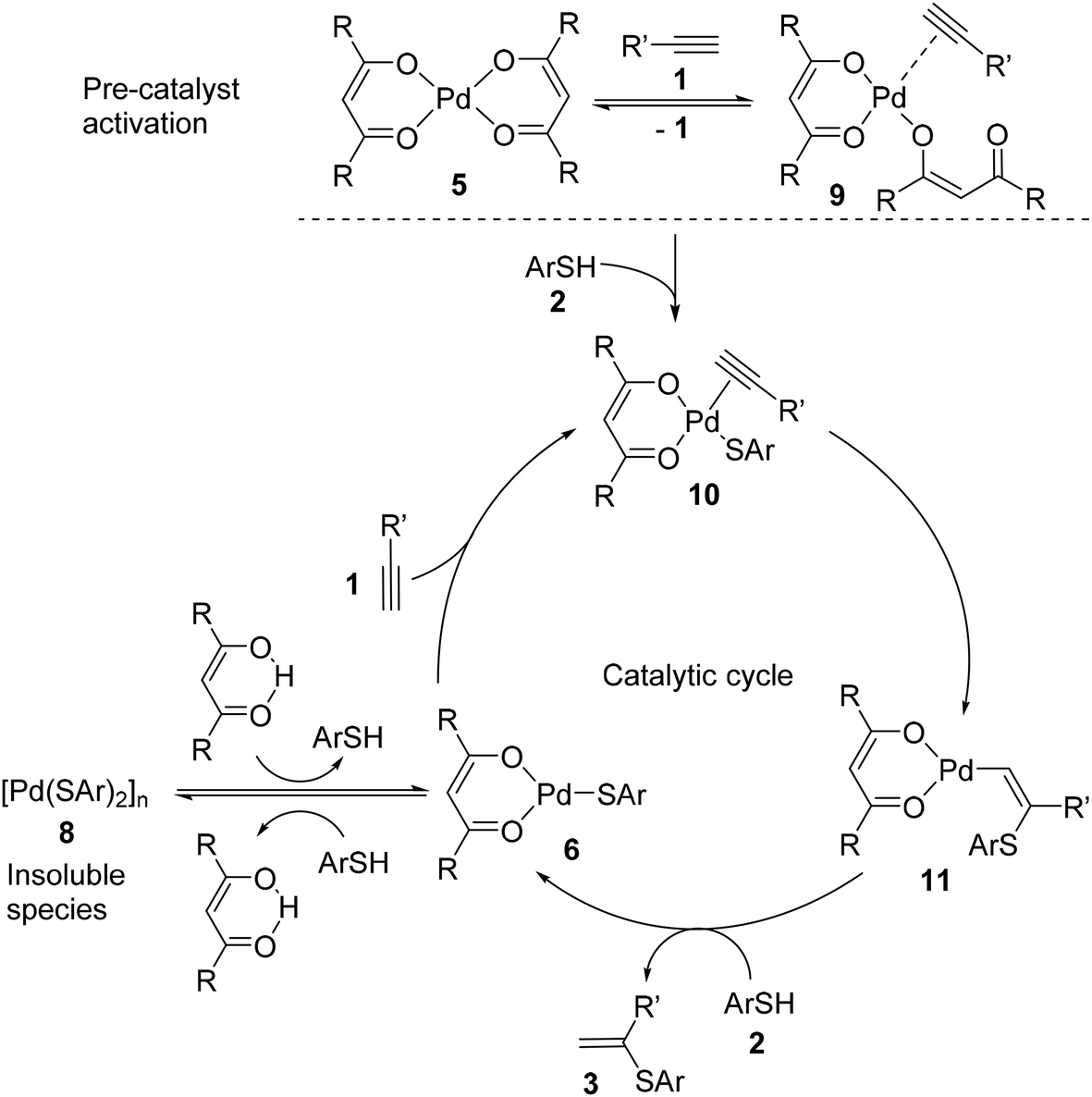
Ten-fold boost of catalytic performance in thiol–yne click reaction enabled by a palladium diketonate complex with a hexafluoroacetylacetonate ligand ... - Catalysis Science & Technology (RSC Publishing) DOI:10.1039/C8CY00173A
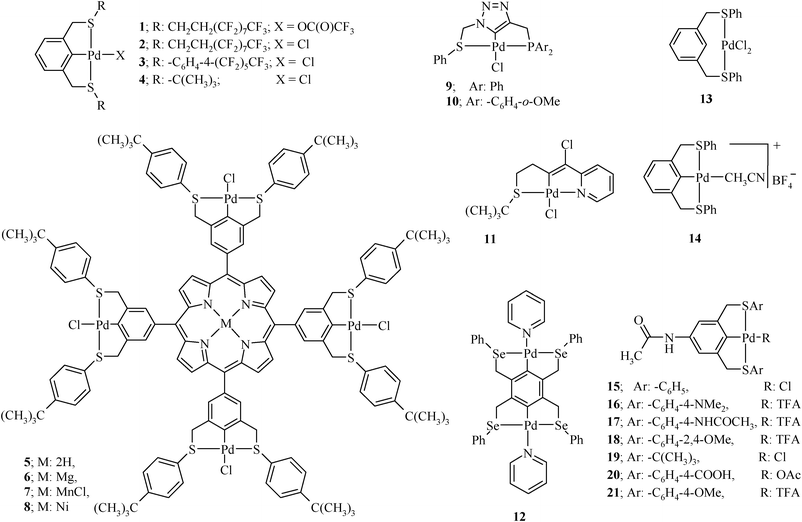
Organochalcogen ligands and their palladium( ii ) complexes: Synthesis to catalytic activity for Heck coupling - RSC Advances (RSC Publishing) DOI:10.1039/C2RA20508D
Synthesis of Alkanethiolate-Capped Metal Nanoparticles Using Alkyl Thiosulfate Ligand Precursors: A Method to Generate Promising

Palladium-catalyzed carbon-sulfur or carbon-phosphorus bond metathesis by reversible arylation | Science
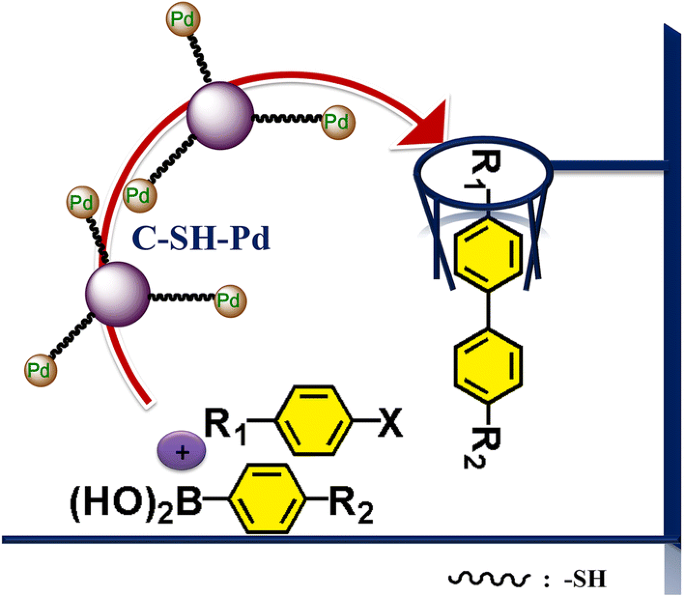
Palladium Nanoparticles Anchored on Thiol Functionalized Xylose Hydrochar Microspheres: An Efficient Heterogeneous Catalyst for Suzuki Cross-Coupling Reactions | SpringerLink
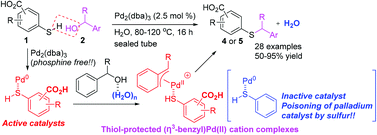
Mercaptobenzoic acid-palladium(0) complexes as active catalysts for S-benzylation with benzylic alcohols via (η3-benzyl)palladium(ii) cations in water - Organic & Biomolecular Chemistry (RSC Publishing)
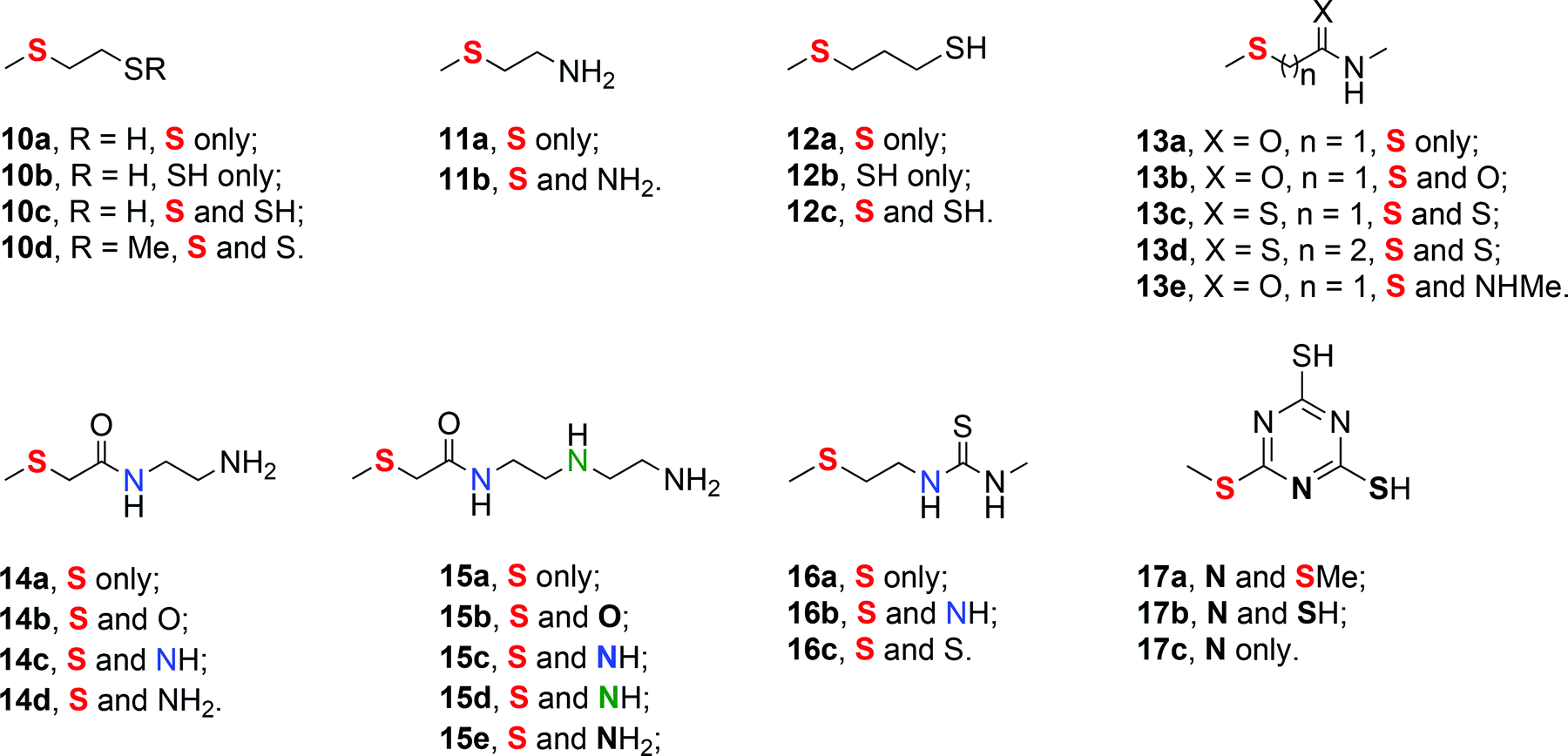
Towards a quantitative understanding of palladium metal scavenger performance: an electronic structure calculation approach - Dalton Transactions (RSC Publishing) DOI:10.1039/C3DT52282B
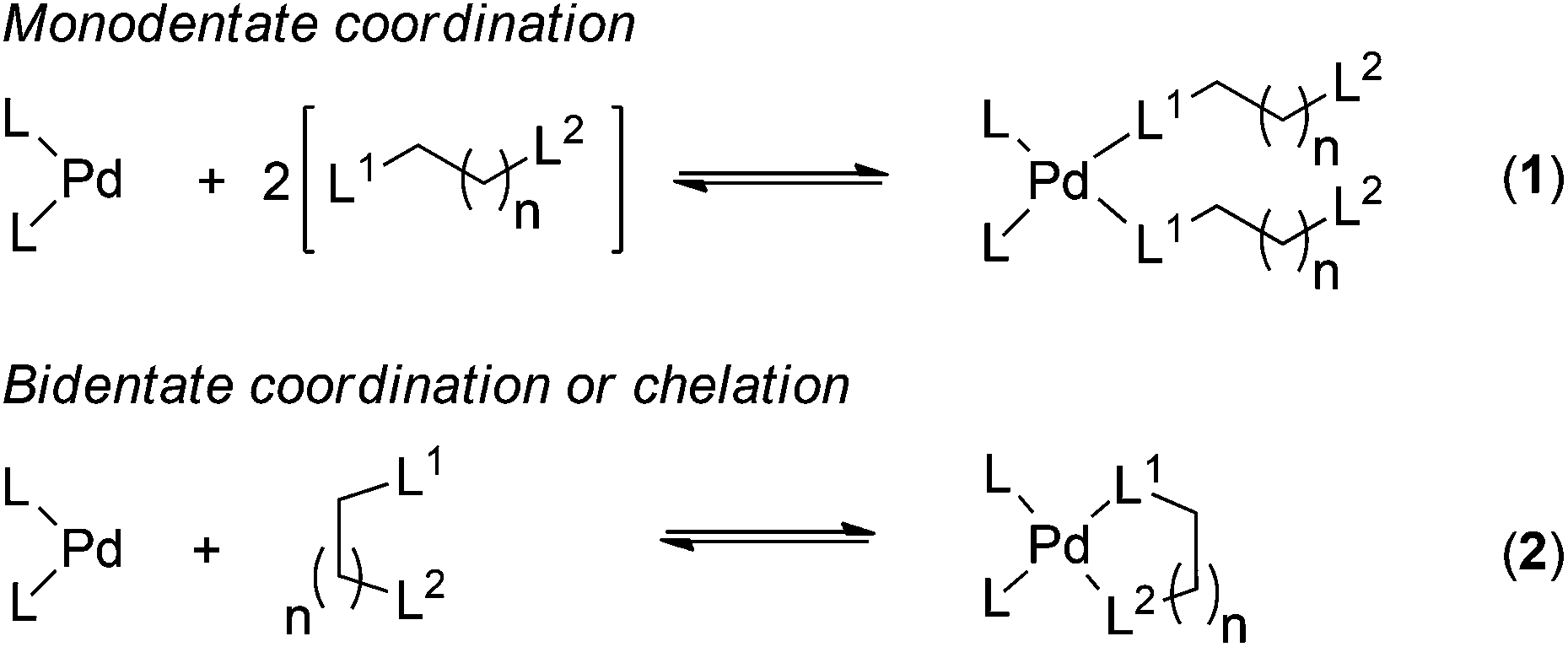
Towards a quantitative understanding of palladium metal scavenger performance: an electronic structure calculation approach - Dalton Transactions (RSC Publishing) DOI:10.1039/C3DT52282B