Dimetallic Palladium‐NHC Complexes: Synthesis, Characterization, and Catalytic Application for Direct C−H Arylation Reaction of Heteroaromatics with Aryl Chlorides - Lee - 2020 - Advanced Synthesis & Catalysis - Wiley Online Library
![Unexpected photochemical transformation of imidazole derivatives containing the 5-hydroxy-2-methyl-4H-pyran-4-one moiety. Environmentally friendly method for the synthesis of substituted imidazo[1,5-a]pyridine-5,8-diones - Tetrahedron Lett. - X-MOL Unexpected photochemical transformation of imidazole derivatives containing the 5-hydroxy-2-methyl-4H-pyran-4-one moiety. Environmentally friendly method for the synthesis of substituted imidazo[1,5-a]pyridine-5,8-diones - Tetrahedron Lett. - X-MOL](https://xpic.x-mol.com/20190826%2F10.1016_j.tetlet.2019.151080.jpg)
Unexpected photochemical transformation of imidazole derivatives containing the 5-hydroxy-2-methyl-4H-pyran-4-one moiety. Environmentally friendly method for the synthesis of substituted imidazo[1,5-a]pyridine-5,8-diones - Tetrahedron Lett. - X-MOL
![Molecules | Free Full-Text | Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives | HTML Molecules | Free Full-Text | Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives | HTML](https://www.mdpi.com/molecules/molecules-22-00399/article_deploy/html/images/molecules-22-00399-sch013.png)
Molecules | Free Full-Text | Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives | HTML
![Molecules | Free Full-Text | Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives | HTML Molecules | Free Full-Text | Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives | HTML](https://www.mdpi.com/molecules/molecules-22-00399/article_deploy/html/images/molecules-22-00399-sch021.png)
Molecules | Free Full-Text | Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives | HTML

US20110028733A1 - Process for the preparation of 5-(2-ethyl-dihydro-1h-inden-2-yl)-1h-imidazole and salts thereof - Google Patents

PDF) Efficient hydroarylation of terminal alkynes with sodium tetraphenylborate performed in water under mild conditions

Imidazole-aryl coupling reaction via CH bond activation catalyzed by palladium supported on modified magnetic reduced graphene oxide in alkaline deep eutectic solvent - ScienceDirect
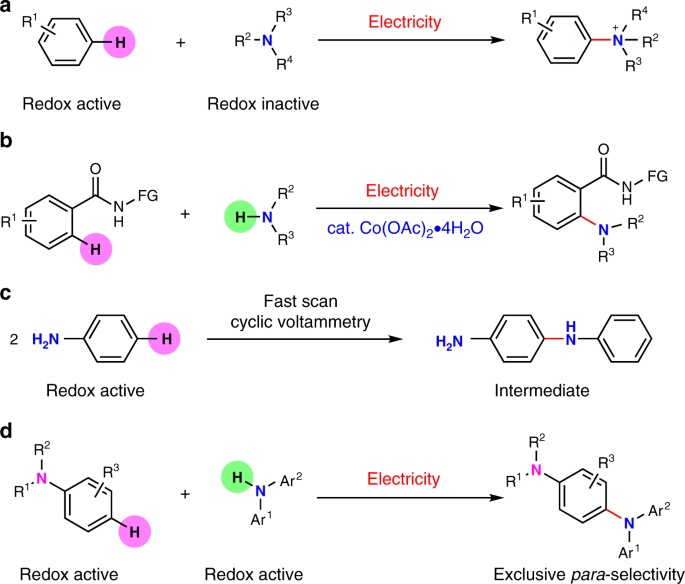
Electrooxidative para -selective C–H/N–H cross-coupling with hydrogen evolution to synthesize triarylamine derivatives | Nature Communications
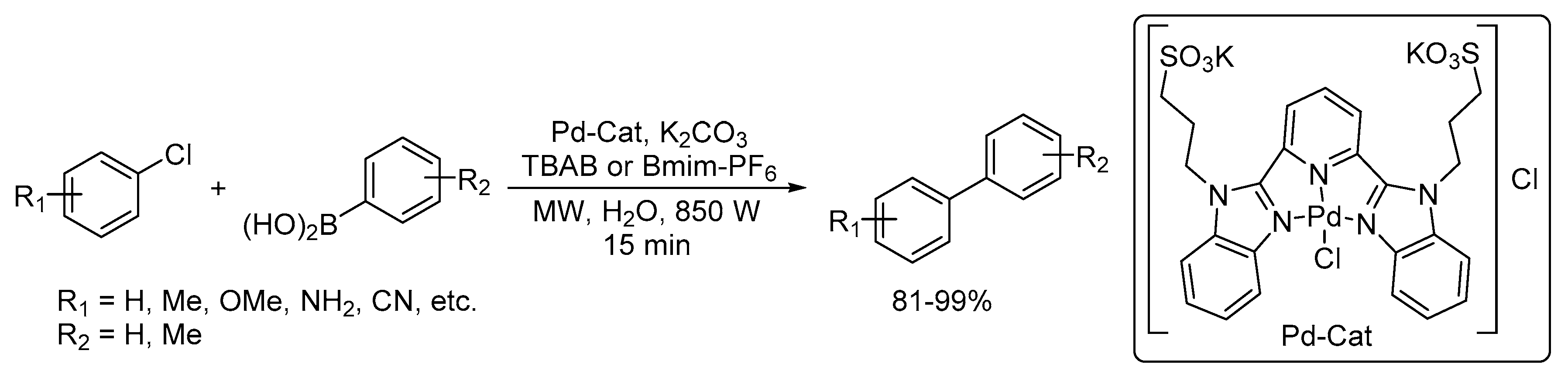
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

Tetranuclear Palladium Complexes of Abnormal N‐Heterocyclic Carbene Ligands and their Catalytic Activities in Mizoroki‐Heck Coupling Reaction of Electron‐Rich Aryl Chlorides - Lee - 2019 - Advanced Synthesis & Catalysis - Wiley Online Library
![Synthesis, Structural Characterization, and Coordination Chemistry of (Trineopentylphosphine)palladium(aryl)bromide Dimer Complexes ([(Np3P)Pd(Ar)Br]2) - Inorg. Chem. - X-MOL Synthesis, Structural Characterization, and Coordination Chemistry of (Trineopentylphosphine)palladium(aryl)bromide Dimer Complexes ([(Np3P)Pd(Ar)Br]2) - Inorg. Chem. - X-MOL](https://xpic.x-mol.com/20190926%2F10.1021_acs.inorgchem.9b02164.jpg)
Synthesis, Structural Characterization, and Coordination Chemistry of (Trineopentylphosphine)palladium(aryl)bromide Dimer Complexes ([(Np3P)Pd(Ar)Br]2) - Inorg. Chem. - X-MOL

Palladium‐Catalyzed C3 or C4 Direct Arylation of Heteroaromatic Compounds with Aryl Halides by CH Bond Activation - Roger - 2010 - ChemCatChem - Wiley Online Library

Are Imidazoles Versatile or Promiscuous in Reactions with Organophosphates? Insights from the Case of Parathion

Molecular structure of C2. Selected bond distances (Å): Pd(1)−C(11)... | Download Scientific Diagram
![Direct arylation and Suzuki-Miyaura coupling of imidazo[1,2-a]pyridines catalyzed by (SIPr)Pd(allyl)Cl complex under microwave-irradiation | El Abbouchi | Mediterranean Journal of Chemistry Direct arylation and Suzuki-Miyaura coupling of imidazo[1,2-a]pyridines catalyzed by (SIPr)Pd(allyl)Cl complex under microwave-irradiation | El Abbouchi | Mediterranean Journal of Chemistry](http://medjchem.com/public/journals/1/cover_article_1124_en_US.png)
Direct arylation and Suzuki-Miyaura coupling of imidazo[1,2-a]pyridines catalyzed by (SIPr)Pd(allyl)Cl complex under microwave-irradiation | El Abbouchi | Mediterranean Journal of Chemistry
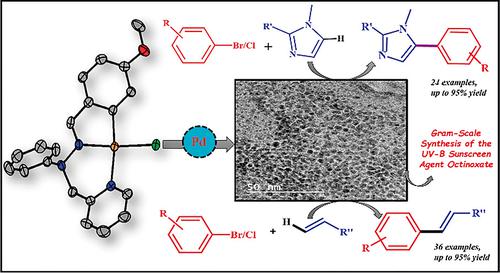
Palladium‐Based Catalysts Supported by Unsymmetrical XYC–1 Type Pincer Ligands: C5 Arylation of Imidazoles and Synthesis of Octinoxate Utilizing the Mizoroki–Heck Reaction - Eur. J. Inorg. Chem. - X-MOL
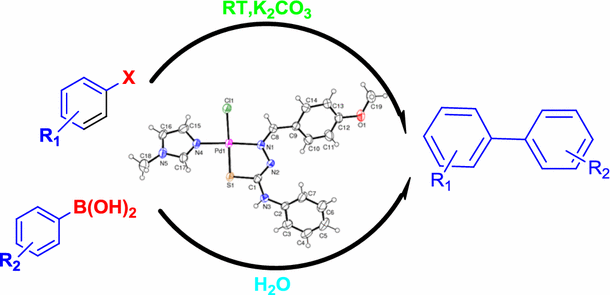
A thiosemicarbazone–palladium(II)–imidazole complex as an efficient pre-catalyst for Suzuki–Miyaura cross-coupling reactions at room temperature in aqueous media | SpringerLink